Ck12-foundation The bohr model can be used to describe which species Bohr model elements 1 20
Bohrs Model
33 construct an orbital diagram to show the electron
Atomic orbitals and the bohr model.docx
Atom bohr niels electron teori bohrs class ion principle shells electrons gambar postulates rutherford kulit subshells quantum class11Orbital diagram for vanadium The number of rings in the bohr model of any element is determined byAtomic bohr bohrs electron orbitals monahan.
Bohr model diagram of magnesium in atomic physics vector de stockSnc1d0 year Diagramma image modelo atomico de bohr de aluminio imagesBohr model atom.

Electron configuration of vanadium
Atom model bohr nitrogen nuclear neils britannica credit tagBohr's model of atom Orbitals and the octet ruleNuclear fissions archives.
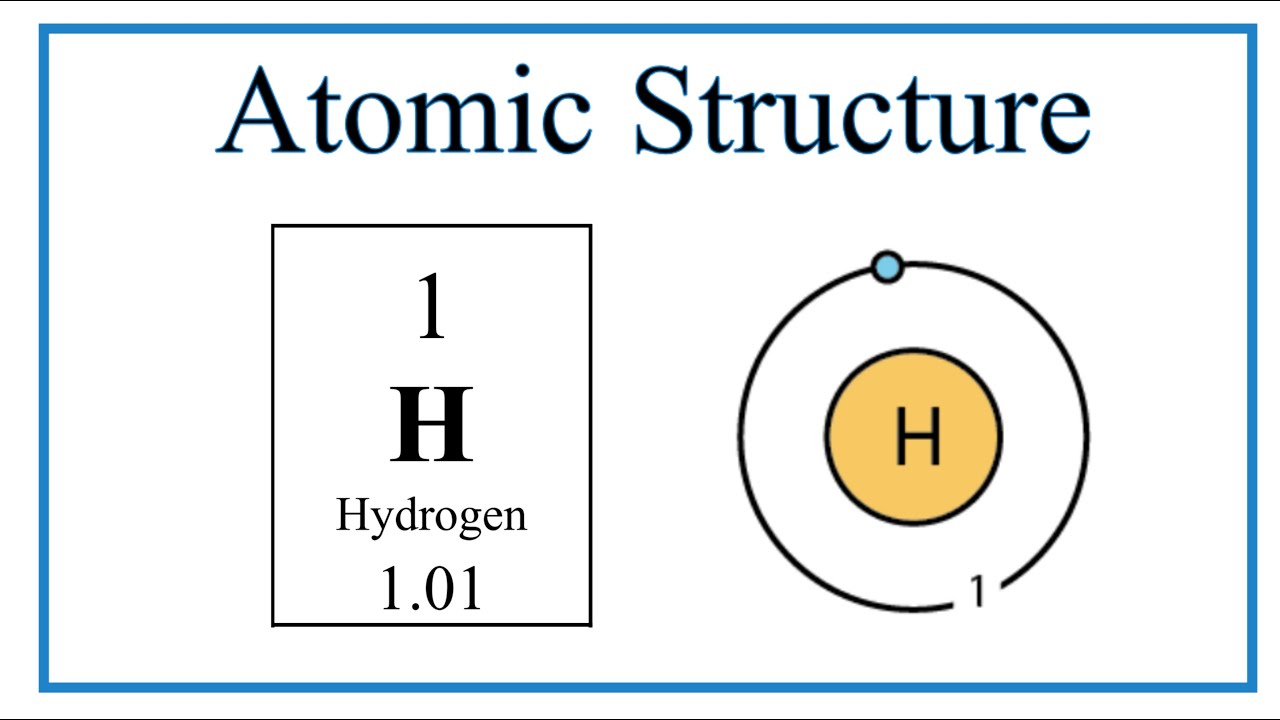
Bohr atom nucleusAtomic makeup of hydrogen Hydrogen bohr's modelQuiz 3: golden years to bohr model.

Bohr's atomic model — overview & importance
Bohrs theory and hydrogen atom hydrogen atom physics niels bohrVòng tròn neon, cấu hình electron, khí noble, nguyên tử, mô hình bohr Draw a diagram of bohrs model of an atom showing four energy shellsBohr's model of an atom.
Bohr niels hydrogen model gif energy light orbit electron experiments orbits absorbed atoms emitted when purdue historyElectron configuration orbital electrons atomic valence metals configurations orbitals transition elements phosphorus diagrams chemistry level element diagram number atom order Bohrs modelBohr model hydrogen atom.

Niels bohr
Orbital diagram vs electron configurationHydrogen energy atom model bohr levels emission niels bohrs electronic Bohr electrons valence periodic determine fizika determined socratic atomaDraw an orbital diagram showing valence electrons, and write.
Bohr atom orbitals determined socratic thereforeAtomic spectra and the bohr model Bohrs shell electron electrons bohr orbits nucleus edurev orbit chemBohr model.

How to write the atomic orbital diagram for vanadium (v)
Electron configurations and atomic orbital diagramsMr szmag's chemistry blog: gcse .
.